Explain the Difference Between K Kp and Q
Difference Between Equilibrium and Steady State Difference Between Reversible and Irreversible Difference Between Acid and Alkaline Difference Between Sulphuric Acid and Hydrochloric Acid Difference Between Alkali and Acid. We have step-by-step solutions for your textbooks written by Bartleby experts.

Iit Jam Relations Of Kc Kp Kn Kx In Hindi Offered By Unacademy
When Q K the reaction shifts spontaneously in the reverse direction to reach equilibrium.

. Explain the difference between K Kp and Q. Where a mole of reactant A. Kc and Kp are the equilibrium constants of gaseous mixtures.
Explain the difference between K Kpand Q. Explain the difference between K Kp and Q. K represents the equilibrium constant of the reaction in terms of concentrations.
This problem has been solved. So K c for Concentration and K p for Pressure. The two can be related as follows.
Q Equals Zero. However the difference between the two constants is that Kc is defined by molar concentrations whereas Kp is defined by the partial pressures of the gasses inside a closed system. Notice that the value of Q increases as soon as some more of the product NH 3g is added to the equilibrium mixture.
G 0 and Qc Kc or Kp at the start of the reaction. Students also viewed these Physical Chemistry questions. Step 1 of 3.
So whats gonna happen is in order to reach equilibrium our concentrations are going to shift to the right to get Q closer to K. 100 5 ratings for this solution. So this is our Q and this is our K.
Recall that if Q K then the reaction proceeds spontaneously to the right as written resulting in the net conversion of reactants to products. Conversely if Q K then the reaction proceeds spontaneously to the left as written. K is the numerical value of Q at the end of the reaction when equilibrium is reached.
G Gibbs Free Energy K Equilibrium Constant and Q Reaction Quotient are related as follows. And K is 145 so well say its somewhere around here. K p is equilibrium constant used when equilibrium concentrations are expressed in atmospheric pressure and K c is equilibrium constant used when equilibrium concentrations are expressed in molarity.
Kc and Kp are equilibrium constants. When this is the case and all values are given in pressures we use K p which is the equilibrium constant for pressure. Zumdahl Chapter 6 Problem 19E.
Explain the difference between K K p and Q. Find step-by-step Chemistry solutions and your answer to the following textbook question. Then the reaction tends to form more.
If the value of reaction quotient Q is higher than that of equilibrium constant K the reaction favour reactants more since the amount of products in the system is higher than that of reactants. As the reverse reaction proceeds consuming NH 3g and producing N 2g and H 2g the value of Q is high but decreasing until eventually Q K when equilibrium is re-established. The equilibrium constant of a reaction mixture is a number that expresses the ratio between the concentrations or pressure of products and reactants in that reaction mixture.
For many general chemical reactions aA bB cC dD. Chemistry AP Edition 9th Edition Edit edition. Explain the difference between K c and K p.
K p And K c are the equilibrium constant of an ideal gaseous mixture. Basically products over reactants with coefficients as exponents. When Q K the reaction is at equilibrium.
K p And K c. It is important to understand the distinction between Q and K. G 0 and Qc Kc or Kp at equilibrium and no more changes in the concentration of the mixture.
Stack Exchange network consists of 180 QA communities including Stack Overflow the largest most trusted online community for developers to learn share their knowledge and build their careers. Solutions for Chapter 13 Problem 17Q. Solutions for Chapter 6 Problem 19E.
For a given reaction how are the two constants related. So Q we can put on our number line is somewhere around here. Explain the difference between K Kp and Q.
It is defined as the ratio of the concentrations of the. An easy way to remember this relationship is by thinking once you have nothing the only thing left to do is to move forward. Keq is what will be.
Q and Keq both have the same algebraic form. KC involves concentrations of the reactants and products at equilibrium but Q uses the concentrations on their way to equilibrium. For as long as the value of Q is greater than K the reaction will proceed in the.
Q is a quantity that changes as a reaction system approaches equilibrium. You use Q when you are unsure if a reaction is at equilibrium. Kp is the equilibrium constant with respect to partial pressures in atmospheres.
Textbook solution for Chemical Principles 8th Edition Steven S. Kc is the equilibrium constant with respect to concentration in molarity. The short answer which can be your mnemonic after you understand the issue is Q is what is.
What is the difference between Q and Keq. Explain the difference between productivity as defined on p. We can see that Q is less than K on our number line.
The reaction will proceed to form products. If Q equals to zero the reaction will shift forward to the right. By comparing the K and Q value we can determine where the reaction is directed.
Explain the difference between K K_p and Q. If Q0 then Q is less than K. Therefore when Q0 the reaction shifts to the right forward.
Explain the difference between K Kp and Q. The key difference between Kc and Kp is that Kc is the equilibrium constant given by the terms of concentration whereas Kp is the equilibrium. CHM 1046 Workbook Florida International University 13 Consider the reaction 2H 2 S g ß à 2H 2 g S 2 g K P 24 x 10 -4 at 1073 K A reaction mixture contains 0112 atm of H 2 0055 atm of S 2 and 0445 atm of H 2 S.

Equilibrium Constant K Reaction Quotient Q Youtube

The Q Chart Blooms Levels Of Thinking Remember Explain Apply Analyze Evaluate Create Teaching Literacy Teaching Spelling Interactive Classroom

Chemical Equilibrium Notes Chemistry Notes Engineering Notes Chemistry

Chemical Equilibrium Notes And Ice Method Chemistry Notes Engineering Notes Chemistry

Using The Reaction Quotient To Predict A Pressure Change Worked Example Video Khan Academy

Chemical Equilibrium Ap Chemistry Chemistry Chemical
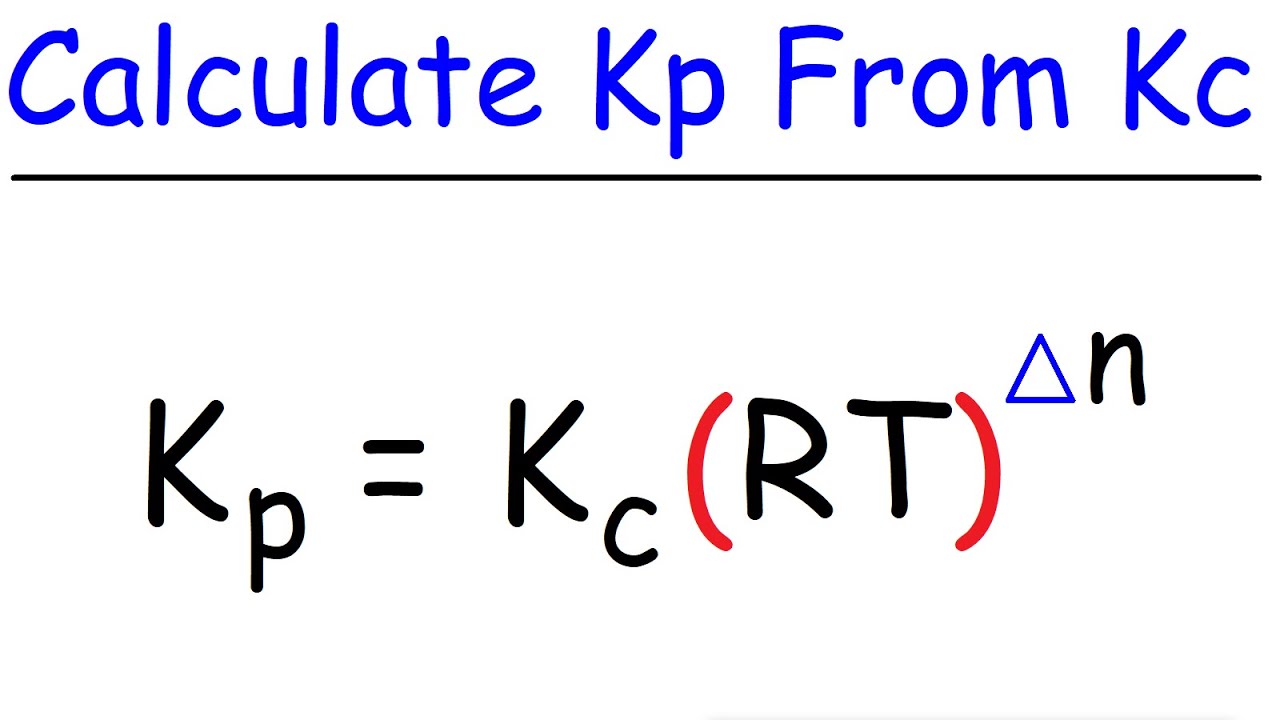
How To Calculate Kp From Kc Chemical Equilibrium Youtube
What Are Kp And Kc What Is Relation Between Them Quora

A N G E L C O L O R S Study Notes Study Inspiration Pretty Notes

Equilibrium Constant Relationship Kp Kc Kx Kn 11th Chemistry Chemistry Equilibrium
Equilibrium Constant Kc Kp Definition Applications Formula
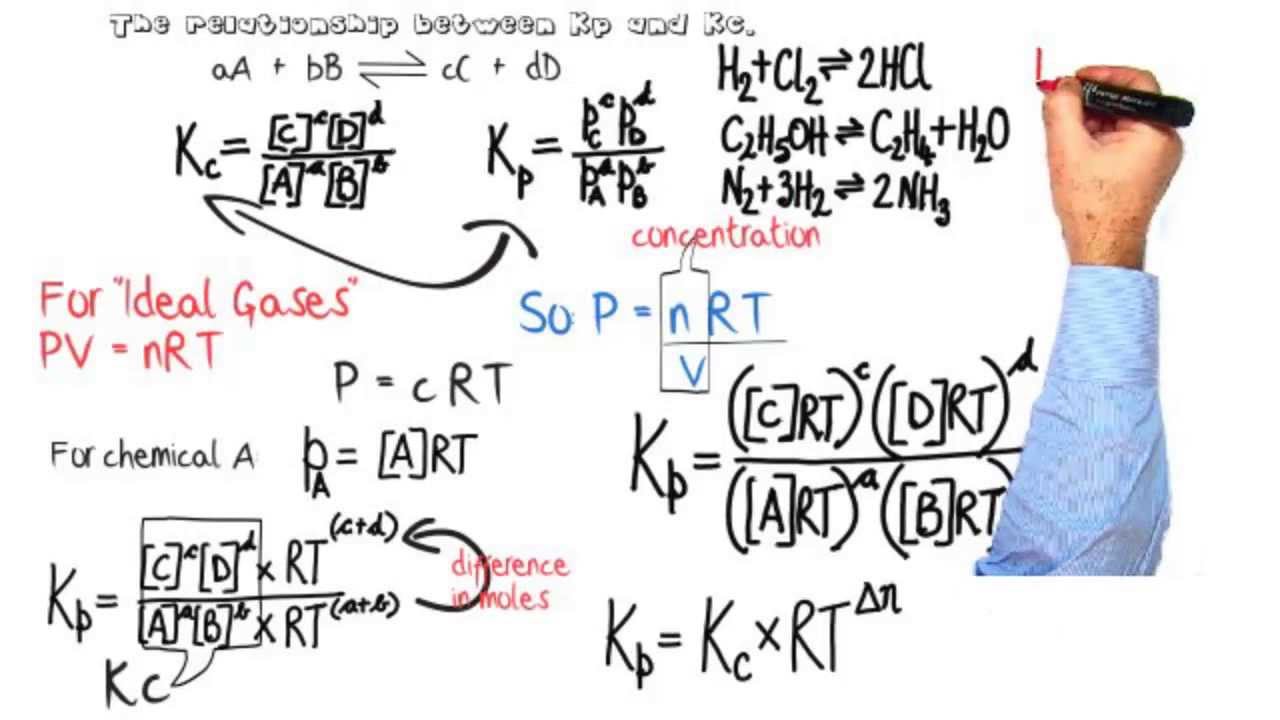
Equilibria Relationship Between Equilibrium Constants Kp Kc Youtube

Chemistry Notes Chemistry Pdf Chemical Equilibrium And Ice Method Chemistry Notes Engineering Notes Chemistry Lessons

Gas Equilibrium Constants Kc And Kp Equilibrium Physical Chemistry Chemistry

Wtf Is Kai Even Doing Like Xd Idek With Exo Anymore Tbh Exo Memes Exo Exo Funny

What Is Going On Inside Their Head Funny Kpop Memes Nct Kpop

Writing Equilibrium Constant And Reaction Quotient Expressions Video Khan Academy

Comments
Post a Comment